Rhizocarpon, english version
Rhizocarpon is a genus of crustose, mainly rock-living lichenized fungi. Many are also lichenicolous, meaning they live as parasites on other lichens, either in an early stage after the spores have germinated in the thallus of the host or persistently in the host. There are around 150 species globally, of which 65 are found in Norway.
- Innhold
- Collecting and determining
- Glossary
The genus was first described in 1805 by A.P. de Candolle. The genus name stems from Greek, Rhizo- (rhiza) meaning “root” and -carpon (karpón) meaning “fruit”, because the apothecia originate from the hypothallus instead of the areoles. This is the first character one looks for in the field when searching for Rhizocarpon species. The hypothallus gives the map lichens their common name, as it resembles the borders of countries when a community of map lichens are growing as a mosaic.
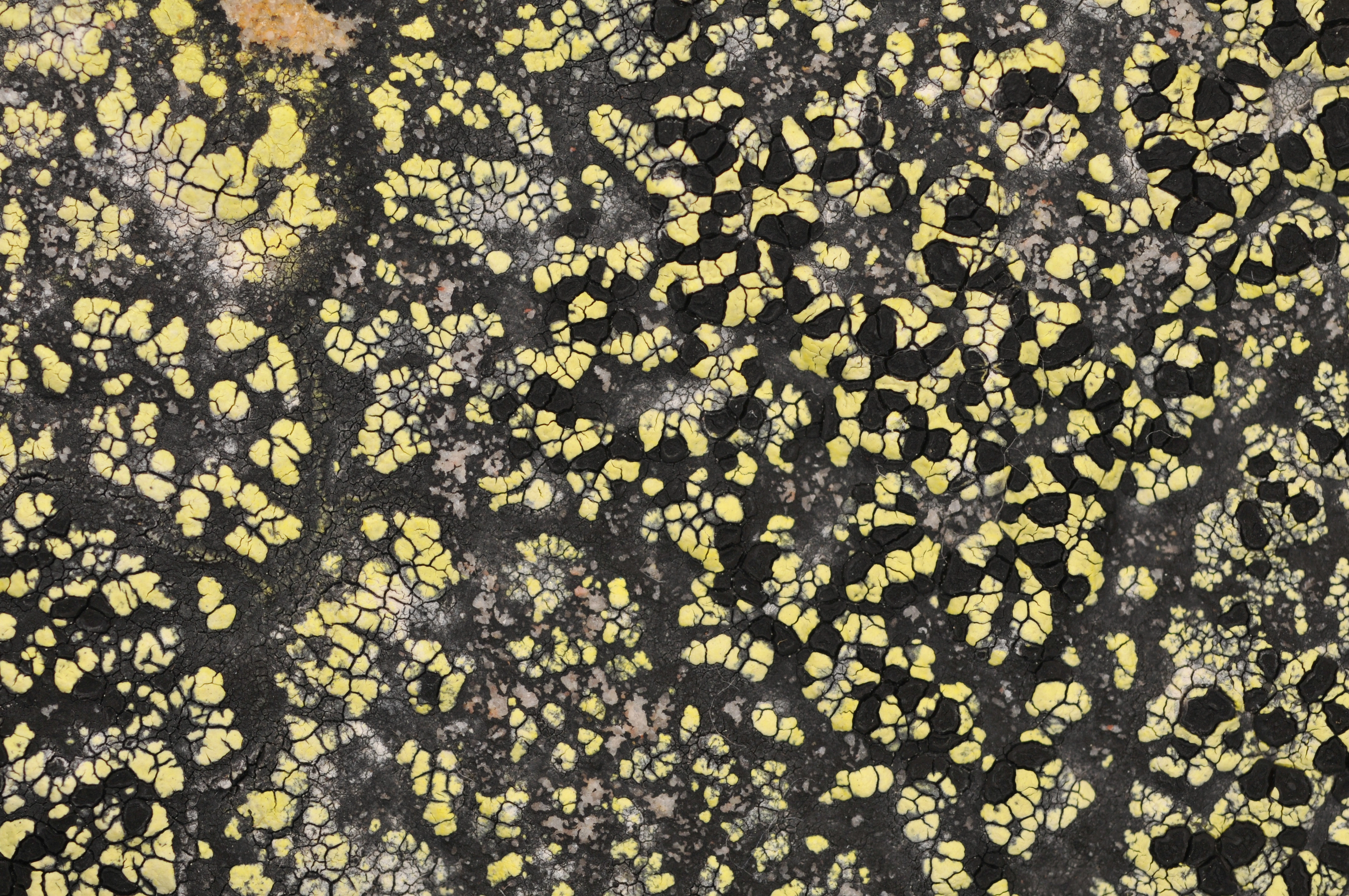
The most well-known of the species is probably the yellow map lichen Rhizocarpon geographicum, which is the type species of the genus and is both widespread and abundant. Locally it can be very abundant, sometimes completely dominating alpine rock surface ecosystems. It may colour mountain slopes yellow by its pigment rhizocarpic acid, often to be spotted kilometres away. However, only around 30 % of the Rhizocarpon species are yellow, and many are brown or grey.
Here, we have written about 63 species of map lichens, but there are still taxonomic uncertainties, cryptic species and putatively undescribed species, so the number of species that exist in Norway must be assumed to be higher. According to the Nordic checklist (Westberg et al. 2021), there are 65 species in Norway.
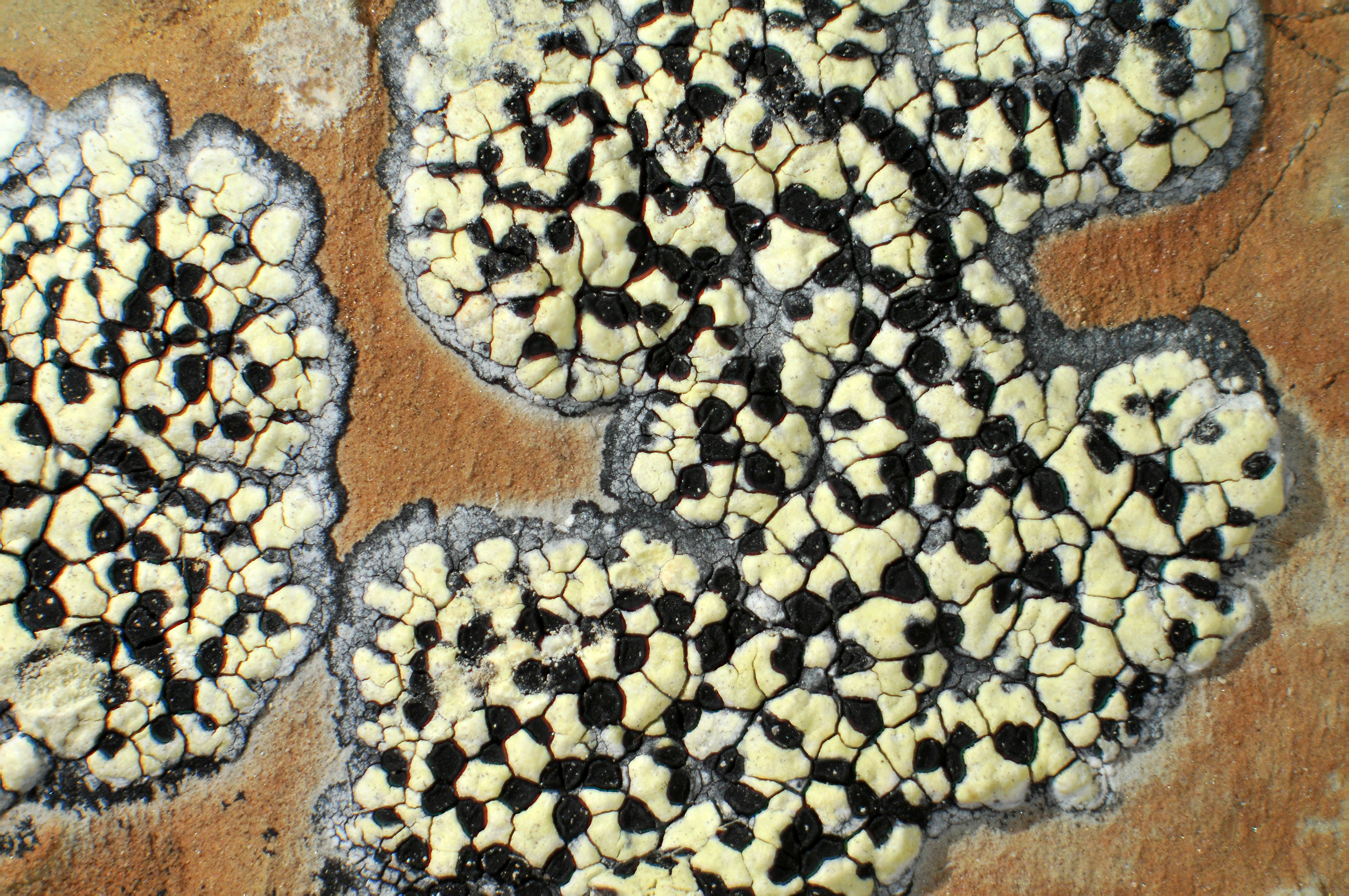
Picture of a map lichen (Rhizocarpon eupetraeum). The red arrows point to areoles (the grey domes). The blue arrows point to apothecia (the black discs). The green arrow points to the hypothallus (the black area surrounding the thallus).
Collecting and determining
To determine Rhizocarpon species, one must often examine them in a microscope. Superficially, many of the species are similar and many of them are variable as well. A very useful character used in species determination is the ascospore morphology. Species vary in spore size, septation, pigmentation and even number of spores in the ascus. Rhizocarpon spores have a gelatinous layer around them called a halo or a perispore. Another useful character is the chemistry. Many lichens make complex chemical compounds, also referred to as lichen substances or secondary metabolites. These can be detected using a method called thin layer chromatography (TLC), but spot testing (with e.g. K, C or Pd) is a simpler and cheaper substitute for some substances. Spot testing the medulla with iodine is also useful, since many species can be separated on the medulla being amyloid or not, meaning whether or not it turns blue/violet. Species can generally be determined by having different combinations of these traits, but it is important to remember that there is a lot of unknown and cryptic diversity and many unresolved species complexes in the genus.
Spot tests can in some cases be conducted in the field, but generally, these methods require that the lichen is first collected. Since Rhizocarpon species grow tightly attached to rock surfaces, this is done with a hammer and chisel. When collecting rock-living crustose lichens, it is good practice to take as little as you need as it leaves permanent marks in the terrain.
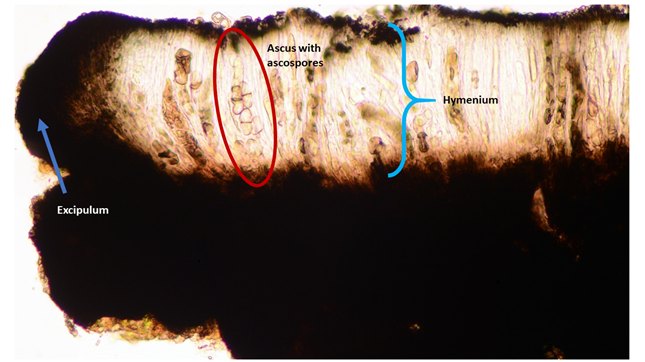
Cross-section of an apothecium in a microscope. The blue arrow points to excipulum, the outer layer of the apothecium. The blue bracket indicates the hymenium, the spore-producing cell layer in the fruiting body. The hymenium consists of asci and paraphyses. The pigmented upper layer of the hymenium is called the epihymenium. The red circle surrounds an ascus with 1-septate, hyaline ascospores. One spore is just escaping the ascus! The thin, most numerous cells between the asci are the paraphyses.
Glossary
Amyloid – if a lichen is amyloid (in the medulla), there is a blue / violet reaction after the addition of a drop of iodine.
Apothecium – fruiting body in many ascomycetes (and therefore most lichens, since 99 % of all lichens are ascomycetes).
Areole – a part of the thallus that contains both fungal tissue and algae cells (see picture). The outer layer is called the cortex (bark). The inner layer is called the medulla (marrow).
Ascospore – sexual spores in ascomycetes.
Ascus – spore-producing cell in hymenium.
C – calcium hypochlorite, used for spot tests to detect lichen acids.
Cortex – the outer layer of an areole, consisting only of fungal tissue. The algae are often located just below the cortex.
Crustose – a growth form, means that the lichen grows densely attached to a substrate, without undergrowth and attachment threads.
Epihymenium / epithecium – upper layer of hymenium. May contain pigments and / or crystals.
Excipulum – the outer layer of the apothecium.
Halo – gelatinous layer that surrounds ascospores in Rhizocarpon. Also called perispore.
Hymenium – the spore-producing cell layer in an apothecium. Consists of sterile paraphyses and fertile, spore-producing asci (singular: ascus).
Hypothallus – a sterile mat of fungal tissue that grows under the thallus, closest to the substrate. Important character for Rhizocarpon, because the areoles and fruiting bodies originate from the hypothallus, but is also found in many other crustose lichens.
Hypothecium – the layer under hymenium in an apothecium.
Iodine – used to detect amyloidity in lichen.
K – potassium hydroxide, 10 %, used for spot tests to detect lichen acids.
Medulla – the inner layer of an areole, below the cortex and algal layer.
Paraphysis – sterile protective / supportive cell threads in hymenium.
Pd – para-phenylenediamine, used for spot tests to detect lichen acids. Carcinogenic, use with caution!
Perispore – gelatinous layer that surrounds ascospores in i.e. Rhizocarpon. Also called halo.
Saxicolous – literally "rock dweller", which means that the lichen lives on rock substrates.
Septum – internal cell wall in a spore, important character in Rhizocarpon.
Thallus – the "body" of lichen, consists of fungal tissue and algae cells.
TLC – thin-layer chromatography, a method for detecting secondary metabolites in lichen.
References
Westberg M, Moberg R, Myrdal M, Nordin A & Ekman S (2021). Santesson’s Checklist of Fennoscandian Lichen-Forming and Lichenicolous Fungi. Uppsala University: Museum of Evolution.