Arthoniaceae, English version
Arthoniaceae is a large family of crust forming lichens, lichen-growing fungi or non-lichenized bark fungi. Approximately 850 species are known from tropical to arctic-alpine habitats worldwide. Seventy-six species are reported for Norway. Lichen-forming Arthoniaceae comprise several rare, red-listed species that are characteristic for humid old-growth forest habitats or the Norwegian rainforests. Only lichens and non-lichenized bark fungi in Arthoniaceae are currently treated here.
Description
Thallus
The thallus is crust-forming. In most species it is not differentiated into a separate bark layer, photobiont layer and medulla. The thallus of the Norwegian species is indistinct to well-developed and immersed in the substrate to superficial. The margin is either not determinate or delimited by a thin brown line. The photobionts, when present, belong to the family Trentepohliaceae or they are a species of chlorococcoid algae.
Fruitbodies
The fruitbodies of the Arthoniaceae are apothecia. The apothecia are variously shaped, but often patch-like (maculate) to clearly elongated (lirellate), branched or star-shaped. They are flat or convex, and immersed in the thallus, raised or attached to the thallus surface. The color is a shade of orange-brown, red-brown, brown black or black. In a few species, the surface of the apothecia is covered in a white, orange or red crystal layer (pruina).
Characteristic for the family is the lack of a distinct, well-differentiated apothecial margin. A few exceptions have been discovered in recent years through molecular analyses. In Norway, such species include Arthonia atra, Arthonia calcarea, Arthonia excipienda and Arthonia granitophila, all with a brown-black (carbonized) apothecial margin.
The spore forming layer (hymenium) of the Arthoniaceae is built of branched and netted hyphae (paraphysoids) that are usually embedded in a colorless to light pigmented gel matrix. The tips of the paraphysoids are often widened and pigmented. In most species, they form a distinct layer (epithecium) on top of the hymenium. The tips of the paraphysoids in the epithecium are either more or less perpendicular or they extend horizontally along the surface of the hymenium. The pigmentation is diffuse or aggregated in distinct pigment granules or plaques. The layer below the hymenium (hypothecium) varies from unpigmented to dark brown or orange-red in color.
The spore-producing cells (asci) are of the Arthonia- or the Arthothelium-type. The inner wall-layer is apically thickened (tholus) and usually penetrated by a triangular to rectangular channel (ocular chamber). All parts of the asci remain colorless in I and KI solution (I, KI negative) except for a KI+ blue ring structure in the tholus that is present in most species. The number of spores per ascus varies from 1 to (usually) 8, depending on the species.
The spores of the Arthoniaceae are 2- to multi-celled. They are divided by transverse septa only (transversely septate) or by both transverse and vertical septa (muriform). In both cases, the apical end cell of the spores is enlarged in several species. The spores are persistently unpigmented or they have a brown pigmentation with or without a granular ornamentation when old.
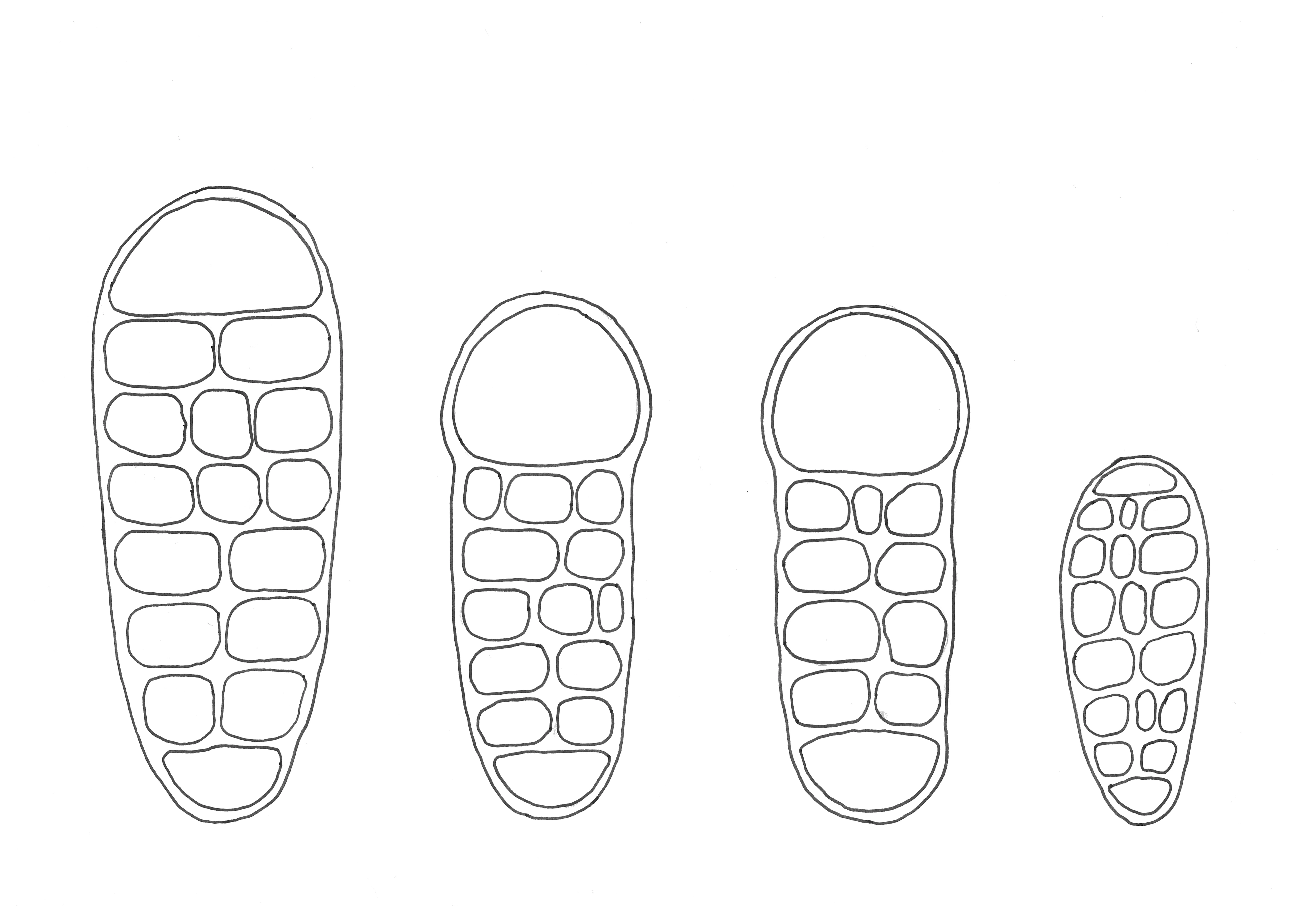
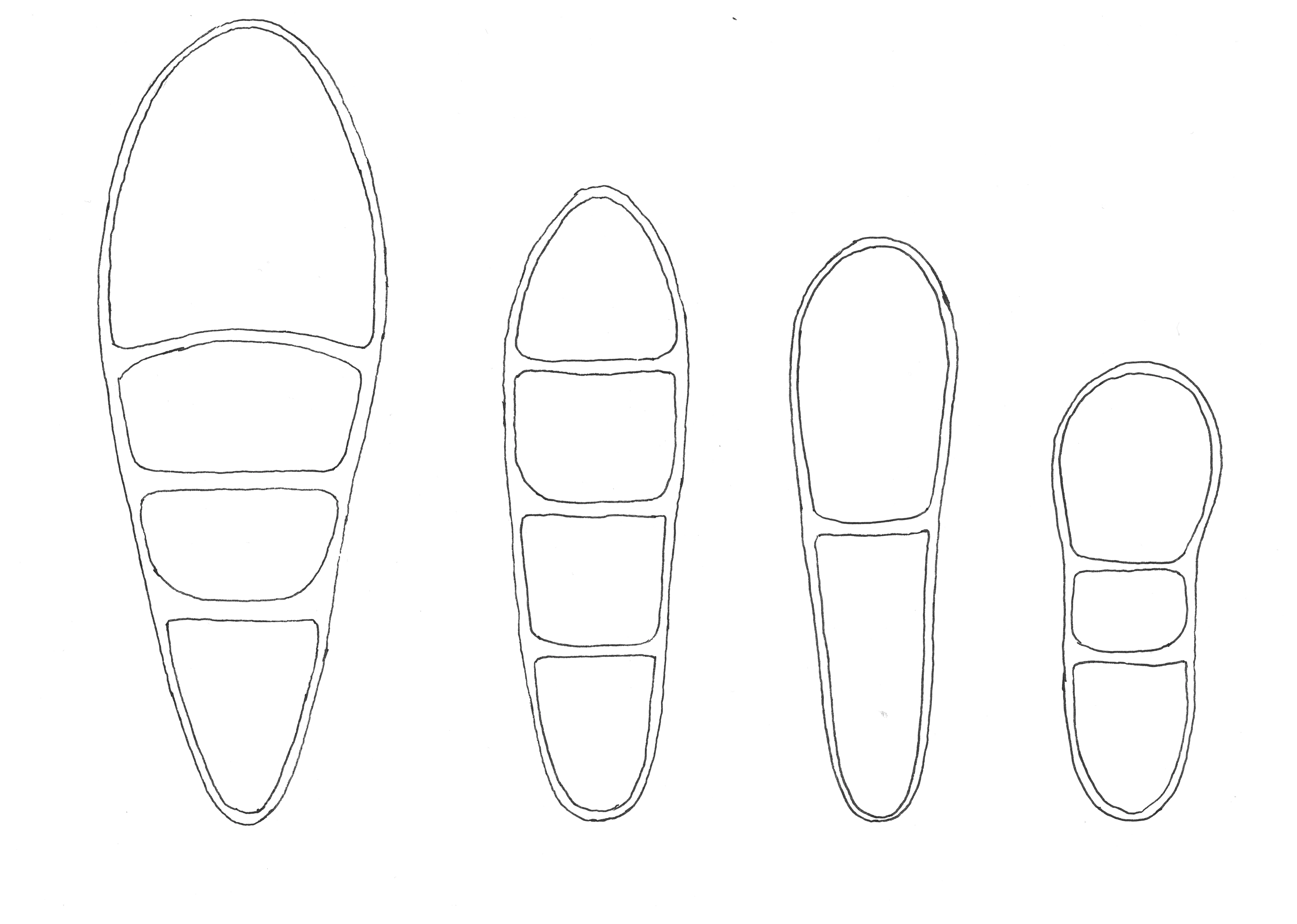
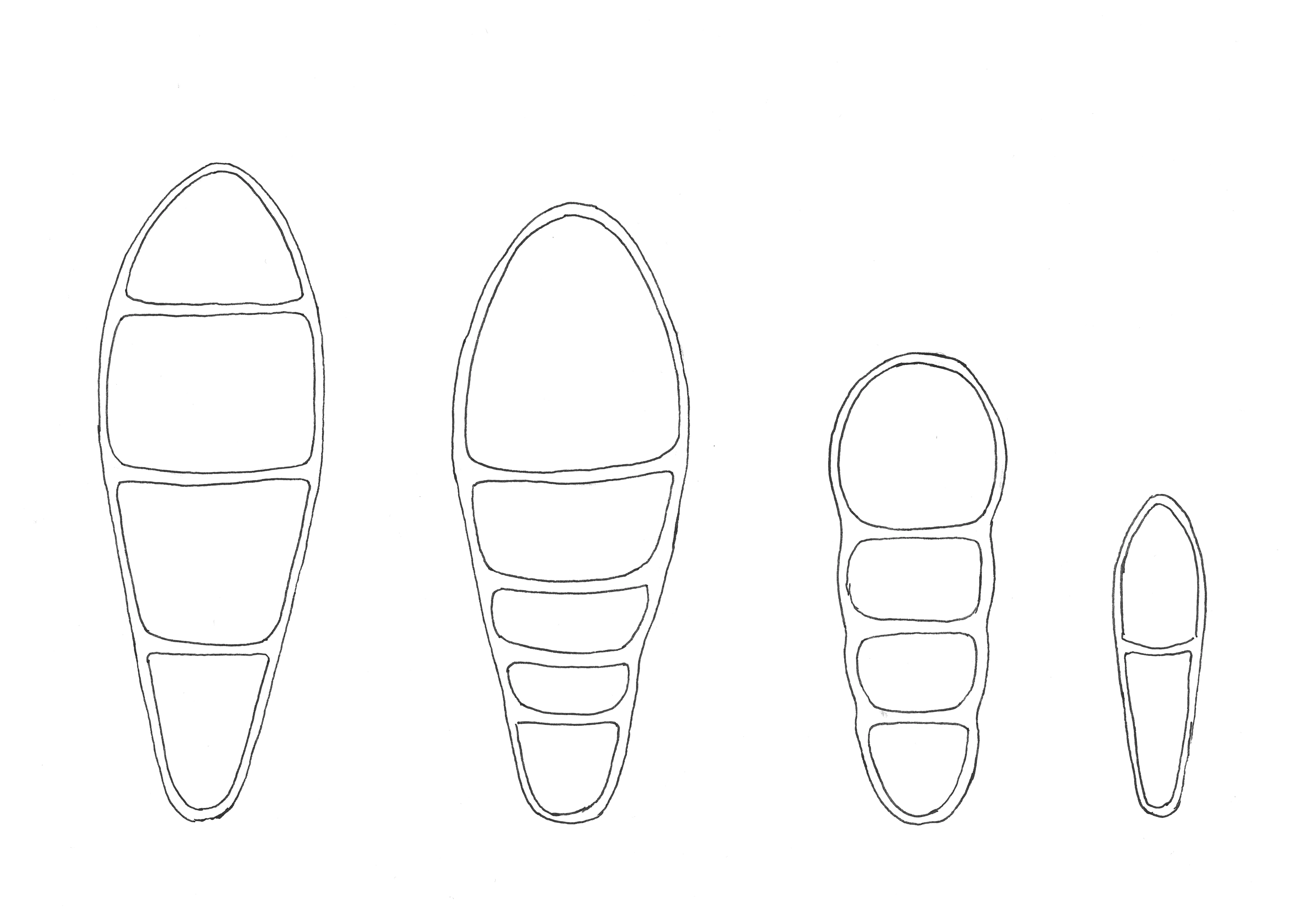
Figure 1. Spore drawings, from left to right: Arthothelium norvegicum, Arthothelium dictyosorum, Arthothelium macounii and Arthothelium ruanum. The spores of Arthothelium dictyosporum are 30 µm long.
Figure 2. Spore drawings, from left to right: Arthonia stellaris, Arthonia radiata, Arthonia apatetica and Arthonia helvola. The spores of Arthonia helvola are 10 µm long.
Figure 3. Spore drawings, from left to right: Naevia punctiformis, Coniocarpon fallax, Reichlingia anombrophila and Bryostigma muscigenum. The spores of Bryostigma muscigenum are 10 µm long.
Anamorph
Asexual, mitotic spores (conidia) of the Arthoniaceae in Norway are produced within pycnidia. The conidia are rod-shaped, ellipsoid or thread-like. Additional conidia-forming structures are known from species of the Arthoniaceae elsewhere.
Chemistry
Many of the Norwegian Arthoniaceae lack lichen secondary compounds in thallus or apothecia. A variety of lichen compounds including depsides and anthraquinone pigments are found in other species. The role played by lichen compounds in the lichen symbiosis is not always known, but important functions have been documented including protection against feeding by snails and insects, anti-bacterial and anti-viral activity, and shielding off excess UV radiation. Lichen compounds are also known to prevent lichen thalli from oversaturation with water, thus facilitating exchange of oxygen and carbon dioxide for photosynthesis.
Identification of lichen compounds is done by means of thin layer chromatography (TLC) or thallus spot tests. For spot testing lichen thalli (or apothecia), simple chemical reagents are applied to the lichens and eventual changes in color are noted. Common reagents used for the identification of lichens include
- K: A 10% solution of potassium hydroxide in water
- C: A solution of calcium or sodium hypochlorite in water; common household bleach is often used
- KC: C applied after K pretreatment
- PD: A solution of paraphenylenediamine in strong ethyl alcohol or clear methylated spirit (freshly prepared)
- I: Lugol’s iodine solution
- KI: I applied after K pretreatment
Testing for the amyloid (I+ blue) or hemiamyloid (I+ red, KI+ blue) color reaction of the apothecial gels is particularly important for classification within the Arthoniaceae.
Ecology
Most lichen-forming Arthoniaceae in Norway are forest epiphytes at low to mid elevations. Large, free-standing trees for example in alleys or pastures represent further important habitats for species of the family. Broad-leaved deciduous trees are the most common substrate, but coniferous trees including Norway spruce (Picea abies) and Scots Pine (Pinus sylvestris) are additionally or exclusively colonized as well. The family includes pioneer species growing on young trees or twigs with thin, smooth bark as well as old-growth forest specialists of high conservation value. Apart from common and widespread species, the Arthoniaceae include species showing a clear southern distribution in Norway as well as species belonging to the highly oceanic phytogeographical element confined to the boreo-nemoral or boreal rainforests along the Norwegian west coast. Strictly saxicolous species are few. They often grow on sheltered rock faces or underhangs in the forest landscape as well as on anthropogenic substrates (mortar, roof tiles, murals) or in the supralittoral zone of rich to slightly calcareous coastal rocks.
Lichen-growing Arthoniaceae mostly belong to the genus Arthonia. Other than the lichenized species, they are species-rich and widespread in alpine habitats as well. Most species are specific to their lichen host, or to a few closely related lichens. Lichen-growing Arthoniaceae are known from a wide variety of shrubby, leafy and crust-forming lichens on trees, rocks and soil throughout Norway.
Systematics
The Arthoniaceae is the largest of currently seven accepted families in the order Arthoniales. Together with the non-lichenized Lichenostigmatales, it belongs to the fungal class Arthoniomycetes. The delimitation of the Arthoniaceae and its classification into homogeneous, natural groupings is not finally settled and currently under investigation. Several genera including Bryostigma, Coniocarpon, Diarthonis, Felipes, Inoderma, Leprantha, Naevia, Pachnolepia and Reichlingia have been segregated from the large genera Arthonia and Arthothelium in recent years based on morphological characters and molecular phylogenetic analyses. These genera have been newly described, or they represent old genus names that have been taken up again.
Even after these changes, Arthonia and Arthothelium are still heterogeneous in Norway. These two genera are artificially distinguished by characters of their spores, transversely septate in Arthonia species and muriform in Arthothelium. Additional changes in the classification of the Arthoniaceae are to be expected upon further revision that will also affect the Norwegian species.
Several of the species that are treated here in Arthonia are similar in morphology to the genus Bryostigma. Phylogenetic data further show that they are closely related to B. muscigenum, the type species of the genus Bryostigma. Most of the lichen-growing Arthoniaceae in Norway do belong to this groups as well. We keep all these species in Arthonia for the time being, pending a much-needed revision of this lineage of the Arthoniaceae.
Identification keys
Many species of the Arthoniaceae can be named in the field based on their specific habitat and general appearance. In critical cases, microscopical examination of internal structures and identification of lichen compounds is necessary for reliable species identification. The following published identification keys can be used for the identification of Arthoniaceae in Norway. However, none of the identification keys listed below individually includes all species occurring in Norway:
- Arthoniales: Arthoniaceae. Revisions of British and Irish Lichens 1 (Cannon et.al. 2020)
- Svenska Skorplavar (Foucard 2001)
- Die Flechten Deutschlands, Band 1 (Wirth et.al. 2013)
Literature
Cannon P, Ertz D, Frisch A, Aptroot A, Chambers S, Coppins BJ, Sanderson N, Simkin J and Wolseley P (2020). Arthoniales: Arthoniaceae. Revisions of British and Irish Lichens 1: 1–48. [https://britishlichensociety.org.uk/identification/lgbi3]
Foucard T (2001). Svenska Skorplavar. Interpublishing, Stockholm, 392 pp.
Frisch A, Ohmura Y, Ertz D and Thor G (2015). Inoderma and related genera in Arthoniaceae with elevated white pruinose pycnidia or sporodochia. Lichenologist 47: 233–256.
Frisch A, Thor G, Ertz D and Grube M (2014). The Arthonialean challenge: restructuring Arthoniaceae. Taxon 63: 727–744.
Frisch A, Thor G and Sheil D (2014). Four new Arthoniomycetes from Bwindi Impenetrable National Park, Uganda. Nova Hedwigia 98: 295–312.
Stornes Moen V (2019). Molecular systematics and species delimitation in Coniocarpon and Arthonia punctiformis s.lat. in Norway. Master’s thesis, NTNU, Faculty of Natural Sciences, Department of Natural History, 61 pp.
Sundin R (1999). Phylogenetic and taxonomic studies within Arthonia Ach. (Ascomycetes, Arthoniales). Doctoral dissertation, Department of Bot., Stockholm University.
Sundin R and Tehler A (1998). Phylogenetic studies of the genus Arthonia. Lichenologist 30: 381–413.
Thiyagaraja V, Lücking R, Ertz D, Wanasinghe DN, Karunarathna SC, Camporesi E and Hyde KD (2020). Evolution of non-lichenized, saprotrophic species of Arthonia (Ascomycota, Arthoniales) and resurrection of Naevia, with notes on Mycoporum. Fungal Diversity 102: 205–224.
Wirth V, Hauck M and Schultz M (2013). Die Flechten Deutschlands, Band 1. Ulmer, Stuttgart, 672 pp.